Matter
Substances - matter that always has the same composition and
properties. Compounds and elements are examples of substances.
Elements - The basic unit of matter. Elements cannot be decomposed
by any ordinary chemical means. A sample of an element is composed of atoms with
the same atomic number.
Compounds - substances that are formed by two or more different
elements and can be decomposed by chemical means. Their properties are usually
different from the elements they are composed of.
Mixtures - A mixture of two or substances.
Homogeneous Mixtures - A mixture that is the same throughout. Also
known as a solution. Salt water (NaCl(aq) is an
example of a homogeneous solution).
Heterogeneous Mixtures - A miture which is different throughout.
Soil and sand are examples of hetergeneous mixtures.
Energy
Energy - the ability to do work.
Forms of Energy
Kinetic - energy of motion
Potential - energy due to position
Chemical - energy associated with a chemical change
Heat - energy associated with the temperature of a body or systems
of bodies
Nuclear - energy due to the changes in mass of the nucleus
Law of Conservation of Energy - Energy can never be created or
destroyed. Energy can only be changed from one form to another.
Exothermic Reactions - releases heat into its surroundings. The
products have a less potential energy than the reactants. ∆H is
negative.
Endothermic Reactions - absorbs heat from its surroundings. The
products have a greater potential energy than the reactants. ∆H
is positive.
Temperature - a measure of the average kinetic energy of a
substance.
Thermometer - You need two fixed points (usually the freezing point and
boiling point of water) to make a thermometer.
Celsius - Freezing point of water is 0°C. The
boiling point of water is 100°C.
Kelvin - Freezing point of water is 273K. The boiling point
of water is 373K.
K = °C + 273
In Chemistry we measure heat energy by using Joules.
q = mC∆T
q = Heat Energy measured in Joules (J).
m = Mass of H2O measured in grams
(g).
C = Specific Heat of H2O =
4.2 J ⁄ g · °C
∆T = Change in temperature.
Sample Problem
Remember, there are 1000 Joules in 1 KiloJoule!
How many KiloJoules are required to change the temperature of 100g of
water from 30°C to 87°C?
Change in temp= 87°C − 30°C = 57°C
Specific Heat of water = 4.2 J ⁄ g · °C
mass = 100 g
q = 100g × 4.2 J ⁄ g · °C ×
57°C
q = 2.4 × 101 KJ
Phases of matter
Matter exists in three phases solid, liquid and gas.
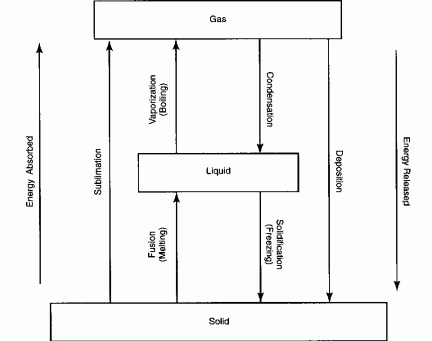
Sublimation - When a substance changes from a solid directly to a
gas. Examples - CO2 and
I2.
Diagram - heating curve
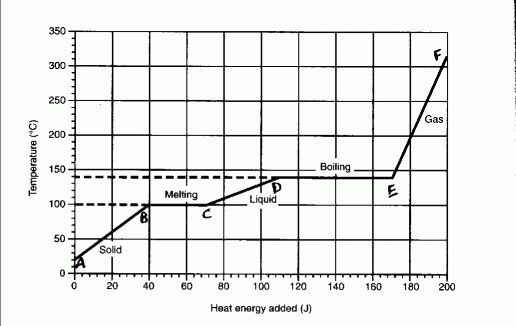
From A to B the substance is in the solid form and it kinetic energy
increases, while its potential energy remains the same.
From B to C the substance is changing from a solid to a liquid at a constant
temperature. This is the only time when solid and liquid phases coexist (solid -
liquis equilibrium). The potential energy of the substance is increasing, while
its kinetic energy remains the same.
From C to D the substance is in the liquid phase and its kinetic energy is
increasing, while its potential energy remains the same.
From D to E the substance is changing from a liquid to a gas at a constant
temperature. The potetial energy is increasing, while its kinetic energy remains
the same. This is the only time when the liquid and gas phase coexist (liquid -
gas equilibrium).
From E to F the substance is in the gas phase and its kinetic energy is
increasing, while its potential energy remains the same.
Gases
Gas Laws
Boyle's Law - as pressure increases volume decreases (an inverse
relationship).
Diagram - Graph of Boyle's law
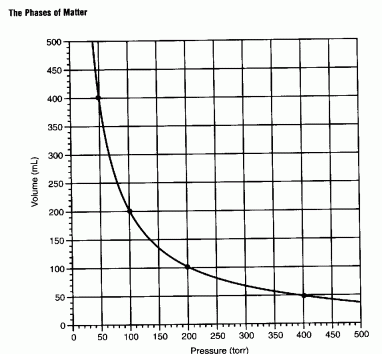
P1V1 =
P2V2
Charles's Law- as temperature increases volume increases (a direct
relationship).
Diagram - graph of charles's law
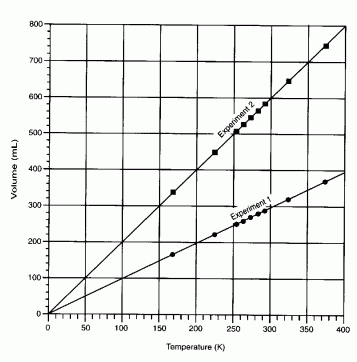
V1 ⁄ T1 =
V2 ⁄ T2
Combined Gas Law
P1V1 ⁄
T1 =
P2V2 ⁄
T2
Standard Temperature and Pressure (STP) - 273K and 101.3kPa or 1 atm
or 760 mmHg or 760 torr.
At STP 1 mole of any gas occupies 22.4L.
Kinetic Theory of Gases
1. All gases move in a continuous random motion in
a straight line path until they are deflected by some force.
2. Collisions cause a transfer of energy but the
overall energy of the system remains the same.
3. The volume of gas particles is negligible
compared to the volume of space in which they move.
4. No forces of attraction are considered to exist
between gas particles. This is called an ideal gas. A real gas would deviate
from this law. The most ideal gas is the gas with the least mass. The gases
which act most like an ideal gas are Hydrogen and Helium.
Liquids
Vapor Pressure - In a closed system the vapor exerts a pressure on
the container and produces the vapor pressure above the liquid.
Boiling Point - As temperature increases so does the vapor pressure
of a liquid. When the vapor pressure equals atmospheric pressure the liquid
begins to boil. The normal boiling point of a liquid is the temperature where
the vapor pressure of the liquid equals standard pressure.
Heat of Vaporization Hv -
heat energy required to change a liquid to a gas.
q = mHv
Hv of
H2O = 2259J ⁄ g
Heat of fusion Hf - heat
energy required to change a solid to a liquid
q = mHf
Hf of
H2O = 333J ⁄ g


