Go to the Answers
Return to Past Chemistry Exams
Site
![]()
1. What is the mass percent HBr (M = 80.91 mol¯1) in an 11.4 m solution of HBr in water?
(A) 48.0
(B) 25.4
(C) 92.2
(D) 15.3
2. Ethane, C2H6, burns in oxygen to form carbon dioxide and water. How many grams of oxygen are required to burn 3.01 x 1023 ethane molecules?
(A) 1.04 g
(B) 12.0 g
(C) 56.0 g
(D) 104 g
3. A sample of a pure element that has a mass of 1.00 g also contains 4.39 x 1021 atoms. What is the element?
(A) C
(B) Ca
(C) Ba
(D) Au
4. If the Celsius tempertaure is doubled at constant pressure, what will happen to the volume?
(A) It will double.
(B) It will remain the same.
(C) It will decrease by one-half.
(D) Additional information is needed
5. Which is the most metallic element in the fifth period?
(A) Y
(B) Cd
(C) Sn
(D) Sb
6. In which set of elements are the atomic radii most nearly the same?
(A) Na, Mg, Al
(B) Cs, Ba, La
(C) Na, K, Rb
(D) Si, P, S
7. Which compound has the highest percentage of ionic character in its bonding?
(A) NaCl
(B) MgCl2
(C) RbF
(D) LiI
8. In which gas are the attractive forces between molecules strongest?
(A) CH4
(B) CO2
(C) H2O
(D) N2
9. When comparing diamond and graphite, which is not true?
(A) The C orbitals in diamond are sp3 hybrids and those in graphite are sp2 hybrids.
(B) Graphite forms two-dimensional sheets and diamond forms a three-dimensional covalent network.
(C) Graphite is the stable form of carbon at standard temperature and pressure.
(D) The bonds in diamond are stronger than those in graphite.
10. Which decrease(s) from Cl2 to Br2 to I2?
I. Forces between molecules
II. Forces within molecules.
(A) I only
(B) II only
(C) Both I and II
(D) Neither I nor II
11. When a small amount of heat is added to a pure liquid (a single compound) that is just starting to boil, what is the result?
(A) The temperature slightly decreases.
(B) The temperature remains the same.
(C) The liquid becomes superheated.
(D) The temperature increases slightly.
12. Under what conditions is deviation from ideal gas behavior most likely?
(A) High T and high P.
(B) High T and low P.
(C) Low T and high P.
(D) Low T and low P.
13. For a given mass of an ideal gas, if the pressure of an ideal gas is tripled and its temperature (in kelvins) halved, its volume will be
(A) 3/2 of its original volume.
(B) 2/3 of its original volume.
(C) 1/6 of its original volume.
(D) 6 times its original volume.
14. What is the most dense element listed below?
(A) Ba
(B) Pb
(C) Os
(D) Rn
15. What is the volume of CO2 measured at 25 °C and 1.2 atm when 25 g of CaCO3 is totally decomposed?
CaCO3 -----> CaO + CO2
(A) 10.2 L
(B) 2.6 L
(C) 15.3 L
(D) 5.1 L
16. If 500 mL of methane, CH4, effuses through a small hole in 48 s, how much time is required for the same volume of helium to pass through the hole?
(A) 12 s
(B) 24 s
(C) 96 s
(D) 192 s
17. When the stocpcock is opened and equilibrium is established between the two bulbs, what is the total pressure in the system?
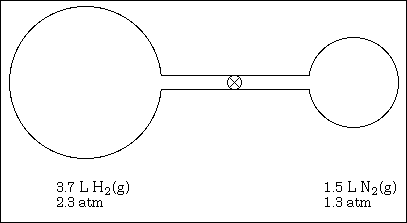
(A) 1.4 atm
(B) 1.8 atm
(C) 2.0 atm
(D) 3.6 atm
18. Which set gives the correct formulas of the most stable forms of nitrogen, phosphorous, and arsenic at room temperature and pressure?
(A) N2, P2, As2
(B) N2, P2, As4
(C) N2, P4, As4
(D) N4, P4, As4
19. Which "hydroxide" is acidic, not amphoteric or basic, in water?
(A) Ca(OH)2
(B) B(OH)3
(C) Be(OH)2
(D) Al(OH)3
20. If Kw is 10¯12 at 100 °C, what is the pH of pure water at this temperature?
(A) 5
(B) 6
(C) 7
(D) 8
21. Which molecule has the shortest nitrogen-to-nitrogen bond length?
(A) N2H2
(B) N2
(C) N2O
(D) N2O4
22. If 5.00 mL of concentrated nitric acid (15.4 M) is diluted to 250 mL, what is the pH of the resulting solution?
(A) 0.51
(B) 0.76
(C) 1.45
(D) 2.89
23. When dissolving rock samples for geochemical analyses, which reagent has the advantage of being both strongly acidic and a good oxidizing agent?
(A) HNO3
(B) HCl
(C) H3PO4
(D) H2SO4
24. When the weak base ammonia is added to a solution containing Fe3+, Cu2+, and Co2+, only the Fe3+ forms a precipitate. Why?
(A) It is only insoluble hydroxide of the three.
(B) Co2+ and Cu2+ form soluble hydroxide complexes.
(C) Co2+ and Cu2+ form soluble ammine complexes.
(D) Only 3+ ions precipitate in basic solutions.
25. Which solution would show the least change in pH upon addition of 3.0 mL of 1.0M KOH? (Assume equal volumes of each solution are used.) Ka = 1.8 x 10¯5 for acetic acid
(A) A solution that is 0.50M acetic acid and 0.50M sodium acetate.
(B) A solution that is 0.10M acetic acid and 0.10M sodium acetate.
(C) A solution that is 1.0M acetic acid.
(D) A solution that is 0.50M sodium acetate.
26. The pH of 0.1M solution of a weak acid is 5.4. What is the Ka of the acid?
(A) 1.6 x 10¯10
(B) 3.2 x 10¯10
(C) 1.6 x 10¯8
(D) 8.0 x 10¯8
27. Black precipitates form in many metal ion solutions when which anion is used as a precipitating agent?
(A) Cl¯
(B) S2¯
(C) PO43¯
(D) CO32¯
28. Hg2CrO4 just begins to precipitate when equal volumes of 0.0004M Hg2(NO3)2 and 0.00002 M K2CrO4 are combined. What is the approximate Ksp value of Hg2CrO4?
(A) 1 x 10¯18 mol/L
(B) 8 x 10¯9 mol/L
(C) 2 x 10¯9 mol/L
(D) 4 x 10¯9 mol/L
29. What is the molar solubility of Pb(IO3)2 in 0.05M NaIO3? (Ksp = 2.6 x 10¯13)
(A) 1.0 x 10¯10
(B) 5.2 x 10¯12
(C) 6.4 x 10¯5
(D) 2.0 x 10¯9
30. Addition of NH3 solution to AgCl(s) will cause
(A) no perceptible change.
(B) AgCl to dissolve and form the soluble complex [Ag(NH3)2]+
(C) AgCl to dissolve and form Ag+, (NH4)+, and Cl¯
(D) AgCl to dissolve and form the soluble complex [Ag2(NH3)]2+
31. For the gas phase reaction A2 <===> 2A, Keq = 1 x 105 at 1000 °C. Two moles of A2 are injected into a 5.0-L container at 1000 °C and allowed to reach equilibrium. What is the molar concentration of A2 at equilibrium?
(A) 0.40
(B) 4.0 x 10¯6
(C) 6.4 x 10¯6
(D) 8.0 x 10¯6
32. What is the oxidation number of Pt in K[PtNH3Cl5]?
(A) 0
(B) +1
(C) +2
(D) +4
33. Which element is used during steel production to oxidize impurities such as carbon and silicon?
(A) N2
(B) Cl2
(C) O2
(D) F2
34. Which aqueous cation is not paramagnetic?
(A) Sc3+
(B) Ti3+
(C) V3+
(D) Cr3+
35. Two-half cells are prepared and connected by a salt bridge and a wire. Both have Zn metal electrodes immersed in a solution of ZnCl2. The concentration of Zn2+ is not equal in the two cells. In this complete cell,
(A) no current will flow.
(B) the total amount of Zn slowly decreases.
(C) Zn2+ migrates through the salt bridge to maintain neutrality.
(D) electrons flow towards the cell with higher [Zn2+].
36. Standard Reduction Potentials at 25 °C
| Half-Cell Reaction | E° (volts) |
| Cu2+(aq) + 2e¯ ---> Cu(s) | +0.34 |
| 2H+(aq) + 2e¯ ---> H2 | 0.00 |
| Cr3+(aq) + 3e¯ ---> Cr(s) | -0.74 |
What is the equilibrium constant for the spontaneous reaction between the Cu/Cu2+ and Cr/Cr3+ half-cells at 25 °C?
(A) 4 x 10¯40
(B) 4 x 10+40
(C) 3 x 10¯109
(D) 3 x 10+109
37. What volume of 0.200 M KMnO4 solution is required to oxidize 25.0 mL of 0.400 M FeSO4 in acidic solution?
8 H+ + 5 Fe2+(aq) + MnO4¯(aq) ---> Mn2+(aq) + 5 Fe3+(aq) + 4 H2O(l)
(A) 2.00 mL
(B) 10.0 mL
(C) 25.0 mL
(D) 50.0 mL
38. Calculate the number of hours an electroplating apparatus would have to operate at a current of 3.00 A to plate 25.0 g of chromium from a 0.500 M CrCl3 solution.
(A) 38.7 h
(B) 4.30 h
(C) 12.9 h
(D) 8.59 h
39. A compound used to etch (or dissolve) glass and silicate compounds is
(A) SnF2
(B) HClO4
(C) H2SiO3
(D) HF
40. How many bonds and of what type are present in acteylene?
![]()
(A) five sigma
(B) three pi and two sigma
(C) four sigma and one pi
(D) three sigma and two pi
41. The equilibrium constant for the reaction
Zn + Sn2+ <===> Zn2+ + Sn
is very large . Which of the following sets gives the correct relative strengths of the oxidizing and reducing agents in this reaction?
Oxidizing Agents Reducing Agents (A) Zn > Sn and Sn2+ > Zn2+ (B) Zn < Sn and Sn2+ < Zn2+ (C) Sn2+ > Zn2+ and Zn > Sn (D) Sn2+ < Zn2+ and Zn < Sn
42. The electrolysis of molten sodium bromide yields Br2 by
(A) oxidation at the anode, the positive electrode
(B) oxidation at the cathode, the positive electrode
(C) reduction at the anode, the negative electrode
(D) reduction at the cathode, the negative electrode
43. Which geometry is associated with an atom having sp2 hybrid orbitals?
(A) linear
(B) pyramidal
(C) trigonal planar
(D) tetrahedral
44. What is the hybridization of C in CO2?
(A) sp3d
(B) sp3
(C) sp
(D) sp2
45. Which compound has the largest dipole moment?
(A) CS2
(B) OCS
(C) SF6
(D) CCl4
46. What are the orbital hybridizations of carbon #1 and #2 respectively, in the following molecule?
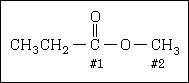
#1 #2 (A) sp3 sp3 (B) sp2 sp2 (C) sp3 sp2 (D) sp2 sp3
47. Which term best describes the shape of the NO2 molecule?
(A) linear
(B) bent
(C) trigonal pyramid
(D) tetrahedral
48. Which substance has the lowest boiling point?
(A) CH3CH2CH2CH2OH
(B) CH3CH2OCH2CH3
(C) CH3CH2CH2CH3
(D) CH3CH2C=OCH3
[Reader's Note: The OH in answer A is attached to the last carbon in the chain. The double-bonded oxygen in D is attached to the second carbon from the right. The original showed these with proper structural diagrams.]
49. A solution of 0.50 grams of an unknown compound creates an osmotic pressure of 4.7 mmHg when dissolved in one liter of water at 27 °C. What is the approximate molar mass (formula weight) of the compound?
(A) 200,000 g mol¯1
(B) 20,000 g mol¯1
(C) 2,000 g mol¯1
(D) 200 g mol¯1
50. Which phenomenon does not provide evidence for surface tension in liquid water?
(A) the formation of a meniscus
(B) capillary action
(C) a nearly spherical raindrop
(D) Brownian motion
51. How many monochloro substitution isomers are possible for normal hexane?
(A) 2
(B) 3
(C) 4
(D) 5
ZnO(s) ---> Zn(s) + (1/2) O2(g)
52. Given that DH° for the reaction is 485 kJ mol¯1, what is the heat of formation, DH°f for ZnO(s)?
(A) -245 kJ mol¯1
(B) -485 kJ mol¯1
(C) +485 kJ mol¯1
(D) The data provided is insufficient
53. The magnitude of the molar enthalpy of vaporization always exceeds that of the molar enthalpy of fusion. The best explanation of this observation is that
(A) vaporization is a more endothermic process than is fusion
(B) more intermolecular bonds are broken in vaporizing a mole of a substance than in melting it
(C) the average kinetic energy of the molecules in a liquid depends on its temperature
(D) None of these explanations suffice
54. Consider the reactions;
H2 + Cl2 ---> 2 HCl
H2 + Br2 ---> 2 HBr
The enthalpy change for the first reaction is more negative than the enthalpy change for the second. Which could best account for this observation?
(A) The Cl-Cl bond is stronger than the Br-Br bond.
(B) The H-Cl bond is stronger than the H-Br bond.
(C) The H-Cl bond is stronger than the Cl-Cl bond.
(D) The H-H bond breaks more easily when it reacts with Cl2 than when it reacts with Br2.
55. A piece of zinc at a temperature of 20.0 °C weighing 65.38 g, is dropped into 180 g of boiling water (T = 100 °C). The specific heat of zinc is 0.400 J g¯1 C¯1 and that of water is 4.20 J g¯1 °C¯1. What is the final common temperature reached by both the zinc and water?
(A) 97.3 °C
(B) 33.4 °C
(C) 80.1 °C
(D) 60.0 °C
56. Given that DG° for the reaction below is -5.40 kJ mol¯1, calculate DG at 298 K when the pressure is 0.50 atm for NO2(g) and 2.0 atm for N2O4(g).
2 NO2 <===> N2O4
(A) -250 J mol¯1
(B) -8800 J mol¯1
(C) -1900 J mol¯1
(D) -11,000 J mol¯1
57. If both DH and DS are negative, the reaction will be
(A) spontaneous at high temperatures, but nonspontaneous at low temperatures.
(B) spontaneous at low temperatures, but nonspontaneous at high temperatures.
(C) spontaneous at all temperatures.
(D) never spontaneous.
58. A large negative DG guarantees
(A) a fast reaction.
(B) a spontaneous rection as written.
(C) a spontaneous reaction in the reverse direction.
(D) None of these are affected by the value of DG
59. Which of the following generally show the largest temperature dependence?
(A) DH
(B) DS
(C) DG
(D) all are temperature independent
60. What is Keq for
HCHO(g) <===> CO(g) + H2(g)
at 700 K given that the values of DG°f are = -113 kJ mol¯1 and -137 kJ mol¯1 for HCHO(g) and CO(g) respectively?
(A) 1.62 x 10¯2
(B) 1.42
(C) 62
(D) 4.53 x 1018
61. If the half-life of a reaction remains the same as the concentration of the reactant increases the reaction could be
(A) first order.
(B) second order.
(C) zeroth order.
(D) none of these.
62. Given these data, what is the overall reaction order for the reaction 2A + B ---> C?
| [A] | [B] | Initial rate of formation
for C (M s¯1) |
| 0.10M | 0.10M | 2.3 x10¯3 |
| 0.10M | 0.30M | 2.1 x10¯2 |
| 0.20M | 0.20M | 1.84 x 10¯2 |
(A) zeroth
(B) first
(C) second
(D) third
63. In the chemistry laboratory, we often run reactions on hot plates or over Bunsen burners. What effect does this increase in temperature have on equilibrium systems in comparison to room temperature?
(A) Higher temperature always increases the rate of reaction and the percentage of products formed.
(B) Higher temperature always increases the rate of reaction but does not affect the percentage of products formed.
(C) Higher temperature affects neither the rate of reaction or the percentage of products formed.
(D) The rate of reaction always increases, but the percentage of products can either increase, decrease or stay the same, depending on the specific reaction.
64. Which statement about the order of a reaction is true?
(A) A second order reaction is also bimolecular.
(B) Increasing the concentration of a reacting species increases the reaction order.
(C) Reaction order can only be determined experimentally.
(D) The overall reaction order can never exceed three since these reactions always occur in smaller steps.
65. Which precipitate is colored?
(A) PbCl2
(B) AgCl
(C) PbCrO4
(D) PbCO3
66. According to the Bragg Equation,
2dsin q = nl
When X-rays of the same wavelength strike two crystals with the same packing but with different atom sizes, the one with smaller atoms will create a diffraction pattern is which the spacing of the points of coincidence
(A) is smaller
(B) is greater
(C) is identical
(D) cannot be determined from the information given
67. In the reaction CH3C(triple bond)CH + 2 Br2 the organic product is
(A) CH3CBr=CHBr
(B) CH3CH=CHBr2
(C) CH3CBr2CHBr2
(D) CH2BrCBr=CHBr
68. Water is the only liquid among the compounds listed below having similar molar mass. Why?
| molecule | molar mass | state at 25 °C |
| HF | 20 g mol¯1 | gas |
| H2O | 18 g mol¯1 | liquid |
| CH4 | 16 g mol¯1 | gas |
(A) Water possesses stronger hydrogen bonds than the other compounds.
(B) Water is more polar than the others.
(C) Water has the highest density of the three
(D) Water has more hydrogen bonds than the others.
69. In the lanthanide elements, which orbitals are only partially filled?
(A) 5s and 4d
(B) 5d and 4f
(C) 6s and 5d
(D) 6p and 5f
70. The quantum numbers for the electron lost when Ag becomes Ag+ are
(A) (4,2,2)
(B) (5,2,2)
(C) (5,0,0)
(D) (4,3,2)
Part II
1. (12%) Anthracene, a compound containing only hydrogen and carbon, occurs in coal tar and is insoluble in water. A student has determined its molar mass to be 185 ± 5 g mol¯1.
(A) Combustion of a 7.500 g sample produces 3.794 g of water. What are the mass percentages of hydrogen and carbon in anthracene?
(B) What is the empirical formula of anthracene?
(C) What is the molecular formula of anthracene?
(D) What is anthracene's molar mass to ± 0.1 g mol¯1?
2. (18%) Lactic acid, CH3CHOHCOOH, is a monoprotic acid that occurs in sour milk. Lactic acid ionizes according to the equation:
CH3CHOHCOOH(aq) ---> CH3CHOHCOO¯(aq) + H+(aq).
(A) Write the Ka expression for this reaction.
(B)(i) Calculate the pH of a 0.45 M solution of lactic acid at 25 °C (Ka equals 8.4 x 10¯4).
(ii) Calculate the pH when 50.0 mL of a 0.45 M lactic acid are combined with 30.0 mL of 0.45 M NaOH
(iii) State whether the solution formed when 50.0 mL of 0.45 M lactic acid is combined with 50.0 mL of 0.45 M NaOH, is acidic, basic, or neutral. Explain your answer.
(iv) Calculate the value of the equilibrium constant K for the reaction of lactate ion with water.
(v) calculate the pH of the solution in iii) above.(C) Using the values from i), ii), v), sketch and label a titration curve for the titration of lactic acid with sodium hydroxide. On the curve, label the buffer region, the equivalence point, and the point where the pKa of the acid is equal to the pH of the lactic acid solution.
3. (14%) For the reaction:
CO2(g) + 2HCl(g) ---> Cl2CO(g) + H2O(g)
(A) Draw the Lewis structures of the reactants and products.
(B) Calculate the DH for the reaction using these bond energies.
| Bond | C-O | C(double bond)O | C(triple bond)O | C-Cl | H-Cl | H-O |
| Bond energy (kJ/mol) |
351 | 715 | 1073 | 331 | 431 | 464 |
(C) Beginning with an equilibrium system at 200 °C and 5.0 atm pressure, indicate whether the reaction will shift to the left (L), shift to the right (R), or remain unchanged (U) when each change is made:
i) Hydrogen chloride is added.
ii) Some Cl2CO is removed
iii) The size of the reaction system is decreased
iv) a catalyst is added
v) The pressure of the system is increased by adding helium gas
4. (12%) Fluorine-18, which decays by emitting a positron, is incorporated in glucose and used to image the brain by positron-emission tomography.
(A) Write a balanced equation for the decay of F-18 by positron emission.
(B) From a consideration of these data, calculate the rate constant for the decay of this isotope.
| t (min) | activity (cpm) |
| 0 | 142 |
| 20 | 125 |
| 40 | 111 |
| 60 | 98 |
| 80 | 87 |
| 100 | 77 |
| 120 | 68 |
| 140 | 60 |
(C) Determine the half-life of F-18.
(D) Determine the time required for the activity of the sample in (B) to decrease to 14 cpm (that is, 10% of its original value).
SECTION B
5. (16%) Write the net equations for each reaction. Use appropriate ionic and molecular formulas for the reactants and products and omit formulas for all ions or molecules that do not take part in a reaction. Write structural formulas for all organic substances. You need not balance the equations.
(A) Solutions of mercury(I) nitrate and potassium iodide are mixed.
(B) A solution of thiosulfate is added to some solid silver bromide.
(C) Two moles of a sodium hydroxide solution are added to a solution containing one mole of phosphoric acid.
(D) An acidic solution of potassium dichromate oxidizes a solution of hydrogen peroxide.
(E) A copper(II) chloride solution is added to some aluminum.
(F) A sodium hydroxide solution is added to some solid ammonium chloride.
(G) Some bromine is added to excess benzene in the presence of FeBr3 as a catalyst.
(H) Some nitrogen(III) oxide is bubbled into water.
6. (8%) Hydrogen forms compounds with all elements except the noble gases. Explain these observations about various hydrides in terms of the types of bonding present.
(A) The hydrides of alkali metals are high-melting solids, whereas the hydrides of halogens are gases at room temperature.
(B) The electrolysis of molten alkali metal hydrides produces hydrogen gas at the anode.
(C) When an alkali metal hydride is dissolved in water, a basic solution results. On the other hand, a hydrogen halide dissolved in water forms an acid solution. (Support your answer with appropriate equations.)
(D) Predict whether HF or HCl will have the higher boiling point and explain your reasoning.
7. (10%) 2-Hydroxybenzaldehyde and 4-hydroxybenzaldehyde are represented by these structural formulas.
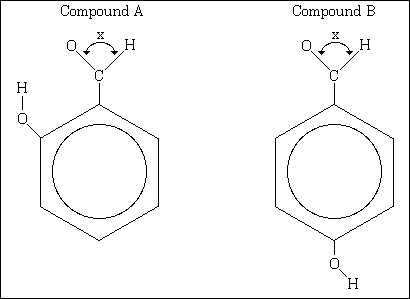
(A) What is the molecular formula for each of these compounds?
(B) For the non-ring carbon atom:i) How large is the bond angle X?
ii) What is the orbital hybridization of this carbon atom? Explain your answers for i) and ii)(C) One of the compounds above has a melting point of -7 °C and the other has a melting point of 116 °C.
i) Which compound has the higher melting point, and why?
ii) Which compound has the lower melting point, and why?(D) Which compound would be expected to have the greater dipole movement? Explain your answer.
8. (10%) The five solid compounds listed below are in separate bottles. The following materials are available for distinguishing the compouds: water, litmus paper, HCl(aq), NaOH(aq) and NH3(aq). Explain how you would distinguish each substance from the others. (Give chemical equations when appropriate.)
(A) AgNO3
(B) CuSO4 (anhydrous)
(C) BaSO4
(D) ZnO
(E) NH4Cl
![]()